Celadon glazes have fascinated ceramics experts for centuries. The technology for producing these glazes, which is shrouded in manufacturing secrets, dates back to around 2000 BC, when these glazes were first used in China.
So why were celadon glazes so easy to produce in ancient times, while today they remain complex for ceramic experts to replicate?
Firstly, this is due to the geological properties of the soil. In the past, ceramicists often dug clay near their homes and kilns were built close to good material sources. The clay itself had a specific composition that, under the right firing conditions, allowed for the creation of the characteristic green and blue tones of celadon glazes. The secret lies in the iron content of the clay or glaze. Iron is a common impurity in many natural raw materials, and ceramicists are familiar with its brown-black color. Generally, iron is removed from raw materials to prevent it from affecting the glaze color or creating unsightly spots on the piece. In the case of pure porcelain, even traces of iron are considered undesirable. However, when iron undergoes a specific firing process, it can create colorful miracles.
Why is it important to study celadon glazes?
As mentioned above, the production of these glazes requires a specific firing process, known as reduction. A reduction atmosphere is an environment in the kiln where oxygen is limited. This effect can be achieved by adding combustible gases (such as natural gas or propane-butane) or wood to the kiln. Traditional kilns in China were often heated with wood or coal, which enabled the necessary reduction process.
The reduction atmosphere is the key factor that enables the creation of celadon glazes. Today, many ceramics experts are capable of producing these glazes, but desriptions of the critical firing process are still few. The production of celadon glazes is still considered to be the result of trial and error. Ceramicists must carefully observe the color of the flame coming from the kiln and then attempt to replicate the process.
The goal of this dissertation is to carefully describe and quantify the firing process, especially the reduction phase, so that atmospheric conditions in the kiln can be repeated with the expected results.
An important factor is also the use of local Czech raw materials.
How to Influence the Color of Celadon Glazes?
To influence the color of celadon glazes, it is crucial to ensure the correct reduction of iron (Fe). The optimal ratio between the oxidation states Fe2+ and Fe3+ should be at least 60% Fe2+ and 40% Fe3+. This ratio can be achieved in several ways.
Reducing iron oxide from Fe2O3 to FeO is essential because these two forms of iron oxide have different properties and effects on the color and glaze.
- Fe2+: This form of iron provides various colors, such as brown, green, and blue tones. It is also known for its ability to create delicate crystalline surfaces or a metallic shine. The presence of ferrous ions in the glaze can contribute to creating unique visual effects.
- Fe3+: This form of iron tends to produce orange, red, and brown shades in glazes. The presence of ferric ions can add visually interesting elements to the glaze.
The correct reduction of iron oxide to both Fe2O3 and FeO states depends on the correct setting of the atmosphere in the ceramic kiln. It is also important to note that iron is very sensitive to changes in atmosphere and the amount of oxygen present during firing. Even small changes can significantly affect the ratio of Fe2O3 to FeO.
Reduction is a chemical process in which the oxidation state of atoms in a compound is reduced. In the case of iron oxide (Fe2O3), this entails the conversion of Fe3+ to Fe2+. This process is directly influenced by the atmosphere in the kiln during firing.
The atmosphere in the kiln can be either a) oxidizing, with enough oxygen to keep ferric ions (Fe3+) in their oxidation state, or b) reducing, with a lack of oxygen, allowing Fe3+ to reduce to Fe2+.
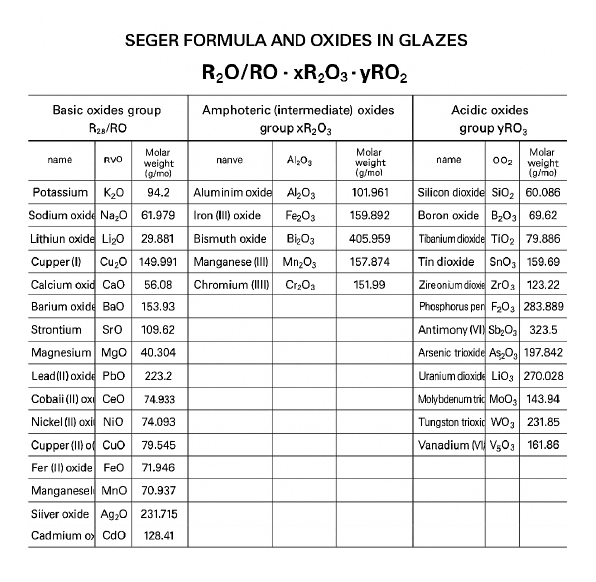
Seger’s Formula
An important tool for creating glazes is Seger’s formula, which includes various chemical components used in glaze production. This formula helps determine the optimal ratio of components to achieve the desired properties of the glaze, such as:
- Silicon Oxide (SiO₂): Used for melting the glaze and forming the glassy layer.
- Aluminum Oxide (Al₂O₃): Provides strength and stability to the glaze, increasing its resistance to melting.
- Alkali Metal Oxides (Na₂O, K₂O): Help reduce the melting temperature and increase the fluidity of the glaze.
- Calcium Oxide (CaO) and Magnesium Oxide (MgO): Improve the glaze’s resistance to mechanical damage and enhance its stability.
By using this formula, it is possible to adjust the glaze so that it creates an optimal environment for the reduction of Fe2O3.
Glaze and its permeability
The glazing process itself is a cornerstone of any ceramics practice, and celadon glazes are no exception. It is an artistic discipline that requires a thorough understanding and mastery of several techniques and processes to achieve the desired results.
Examining the glaze is fundamental to understanding its permeability and absorbency. These parameters are crucial for determining the right temperature for starting the reduction process.
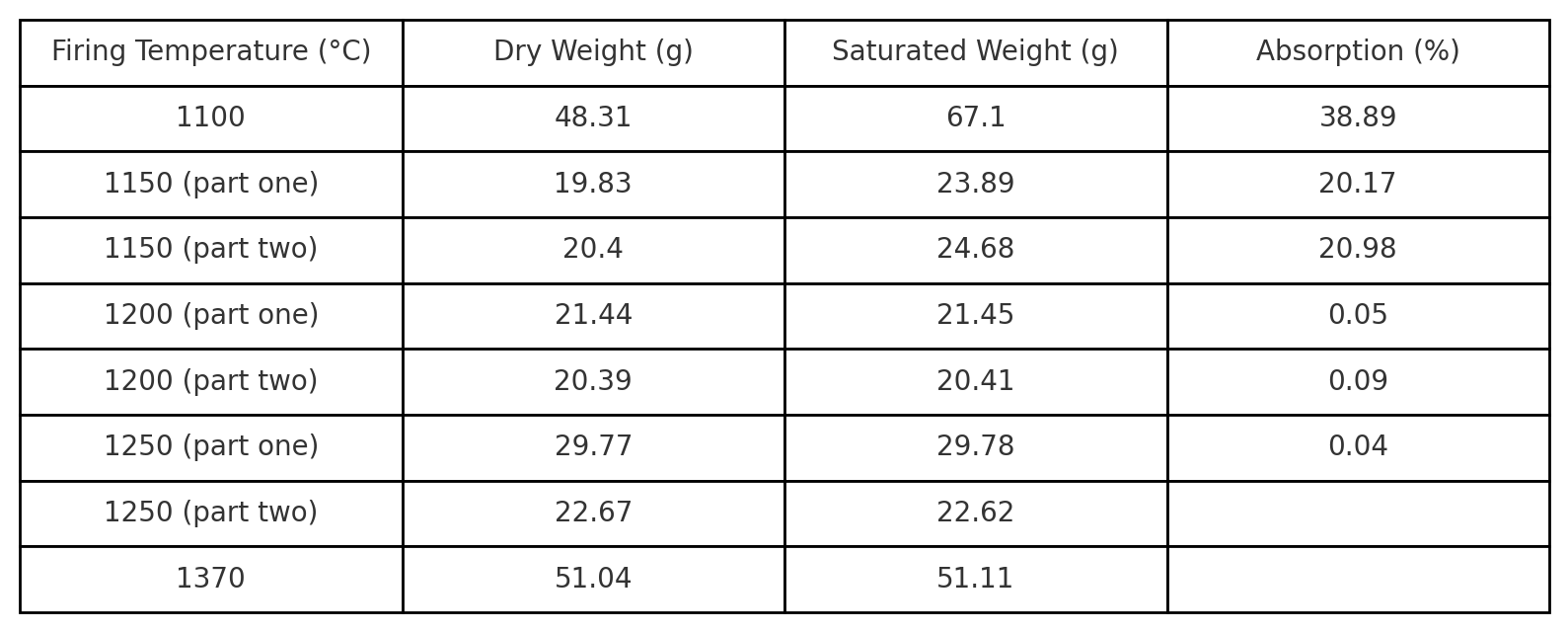
In the research, samples were created from the working glaze. These samples were then fired at specific temperatures to observe how the glaze behaves at various temperature levels. The temperatures were recorded and monitored to ensure future reproducibility.
One of the most important properties of the glaze that can be measured is its absorbency. Absorbency is the degree to which the glaze absorbs moisture. This property is important for determining how the glaze behaves during the firing process and how it will behave after firing. The absorbency of the glaze is usually determined through a test in which the glaze sample is placed in a container of water, and the amount of water absorbed by the glaze is then measured.
By conducting these tests and recording the results in a table, an overview of the glaze properties was obtained. This information is then used for further research and experiments with the glaze, which helps improve glazing techniques and processes.
The analysis of these samples led to important conclusions about the reduction process of the glaze. Research shows that the key factor in this process is the temperature at which the reduction begins. The results indicate that the reduction process must start at around 1000°C, which is crucial for achieving the desired quality of the glaze.
Gas-Electric Firing Kiln
During experiments in commonly available ceramic kilns, it was found that these kilns were unable to create the required atmosphere. Therefore, a laboratory gas-electric firing kiln was designed, combining regulated electric heating with adjustable atmospheric control. This allows for precise control of the atmospheric conditions during firing, which is crucial for achieving the desired effects in celadon glazes.
The kiln combines easily adjustable electric heating controlled by a program controller with controlled atmosphere in the kiln. The new kiln design places heating elements in multi-layer tubes with zero absorbency and permeability, LUNIT 73. This material meets the requirements of Kanthal for ceramic supports with a minimum Al2O3 content of 45%.
Heating tubes pass through the kiln lining and are sealed at the ends with refractory fibers, Sibral Standard, which allow air to reach the heating elements made of Kanthal A1 wire. This air creates an oxidizing protective layer for the Kanthal A1 material. The length of the expanded spiral matches the kiln’s internal dimensions. The correct surface load and spiral pitch ensure that the maximum allowed temperature for Kanthal A1 is not exceeded.
The kiln is fed with a mixture of propane-butane and air in the correct ratio via a Teclu laboratory burner. The amount of propane-butane and air can be adjusted using flow meters. The atmosphere in the kiln is therefore easily adjustable, visible, and reproducible. The exhaust gases are analyzed using a λ-probe made of stabilized zirconium oxide, heated by a constant voltage source of 12.3V to a working temperature of 650°C. The voltage of the probe is measured in relation to the kiln firing curve.
The kiln’s internal dimensions are 200x200x300mm, with a usable space of 150x150x275mm. Temperature is measured using a PtRh10-Pt thermocouple placed in a non-permeable corundum tube to avoid contamination of the platinum with iron compounds. The kiln heating is controlled by a Bentrup program controller.